The MedTech Regulatory, Research &
Intelligence Platform
Medical Devices | In Vitro Diagnostics | Medicines

+100 Million of organised and classified resources up to date
News, Databases, Profiles, Tools, Webpages, Documents, Reports and much more.
The LEADING single-search platform for all e-resources, intelligence, research
Search
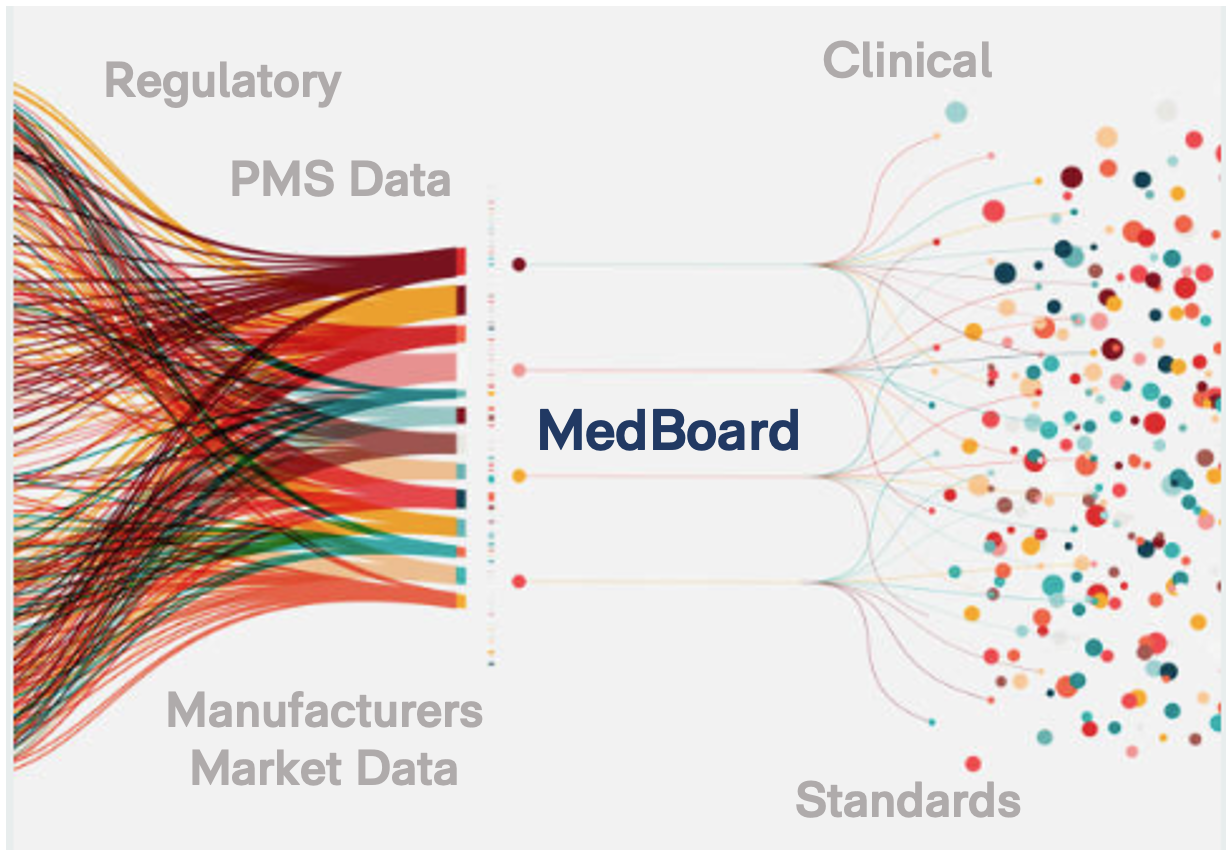
MedBoard Search Engine & Individual Real-Time Databases
Regulatory (+ 200 countries), Market & Clinical News
Public & Trusted Sources
Clinical Literature, Clinical Trials and Guidelines
Worldwide PMS & Recalls
Documents, Regulations, Guidance, Reports, etc.
Approvals, Registrations
and much more
Systematic Review
Regulatory, Market, PMS, Literature, Review Automation
Customization Features
Systematic Review Process
Screening & Appraisal Tools
Advanced Filters
Automated Workflows
Audit trial & Collaborative
PRISMA Flowcharts & Reports
Review Displays
Multiple Export Formats
and much more
Intelligence
Advanced Profiles, Analytics, Summaries, Insights and Tools
Regulatory Intelligence toolkit (+200 countries)
Country Regulatory summaries
Countries, Standards, Products, Manufacturers, Hospitals Advanced Profiles
Evidence AI Classification
Insights, Statistics, Trends
Translations & Trackers
and much more
Portfolio Mgmt
Product Codes, Portfolio, RIM & Country Registrations
For MedTech Manufacturers:
Portfolio Management by product codes and families
Regulatory Impact Reporting
Products Country Registrations
Renewal Management
Notifications & Alerts
Ability to Connect to other platforms
and much more
For All:
Specific for Manufacturers and Developers:
Testimonials and Customer Success Stories
Aroa BioSurgery
Medical Manufacturer “" MedBoard is simply amazing and saves me valuable time and effort conducting research and staying up-to-date on changes around the globe. A monthly report that used to take hours of fumbling around multiple websites and weeding through email newsfeeds now takes no time at all. Absolutely the best value for medical device data !!! ".
Vision RT
Medical Manufacturer “" MedBoard has streamlined our Regulatory Intel and PMS activities, removing the need to spend hours every month trawling regulator websites for information. The platform is user-friendly and the MedBoard team is incredibly responsive".

Zip Diagnostics
IVDs Manufacturer “" We are enjoying using MedBoard very much. The combination of scientific, regulatory and market information in one well organised place is very appealing to us. It has significantly reduced the amount of time and effort. The literature search monitoring and traceability has been very useful. Thank you very much!".
More ACCESSIBLE & AFFORDABLE medical industry information and data, for ALL
We think of the big picture, more accessible information for ALL stakeholders will increase patient safety, innovation, faster execution and quality of life of professionals and patients.
|
|
Built by Medical Professionals that already been working in the MedTech industry for many years, and Powered by Data Science and AI, validated, cloud based, and ready-to-use. We understand the industry, we understand your challenges and we have great passion for innovation.
We think of MedBoard as a MUST TO HAVE for any organization working in the life sciences sector. Every organization, regardless their size, should access to and afford such a resource. Pricing to accessible quality databases that are critical for patient safety and medical professionals should never be a barrier. |
EMPOWERING PROFESSIONALS working in the Medical Sector
We believe in humans, and their expertise and knowledge. The decision making is with you. Our platform, search engines, tools and automated workflows are built to maximise human ability and empower you. And also, in alignment with the expectations of regulators, agencies and notified bodies.
 |
How many medical professionals with many years of experience, degrees, masters, Phds, are manually searching and doing repetitive tasks, when performing reviews or reporting, or even checking the latest updates. This is mostly due to manual and tedious work (searches, reviews and reporting). This is even getting worse and worse over the last years with the unprecedented increase of amount of data shared by Key stakeholders. At MedBoard, we love when we hear this type of feedback: "this used to take me 3 weeks, now it takes me 10 minutes" . We focus on automating and making easier your work and the most cumbersome and complex tasks. |
TRUSTED by leading and world's best life science companies of every size. Hundreds of users.
We use only trusted sources. We keep an incredible 100% retention so far.
 |
Information and data that you can trust. A large number of Manufacturers (Medical Devices, In Vitro Diagnostics, Biotech, Pharmaceuticals), Consulting Companies, Providers, Research Organizations and even Governments and Agencies trust and already use MedBoard. The profile of our customers are agile, think-forward, proactive organizations with great understanding of the challenges and the need for adoption of digital solutions. |
LARGEST Search Engine curated, organised and dedicated for the MedTech industry and Life Sciences
We offer the big picture search but also the granular search in specific databases and resources:
|
+ 100 Million results and growing: • News
• Profiles
• Regulatory, PMS and Clinical databases
• Market databases
• Webpages
• Resources
• Media
|
Many Profiles covering all market, such as:
• Regulatory Authorities: + 225 • Technical Standards Profiles: + 40,000
• Medical Manufacturers: + 50,000
•Medical Products: + 1Million
• Medical Areas: + 100
• Product Types: + 6,000
• Hospitals: + 6,000
• Technical Standards: + 6,0005• Regulatory Requirements: + 50
.
|
|
These databases include additional filters (e.g. evidence type, date, source, etc.), some examples are:
• News - Regulatory Authorities: +50k Updates
• News - Technical Standards: +50k Updates
• News - Clinical & Market , Press Releases, Hospital News: Million of Updates
• Documents: Regulations, Regulatory Guidance, etc: +50k documents
• Clinical Studies & Trials: +600k
• Clinical Guidelines and HTAs: +20K
• FSCAs & Recalls: +80k from more than 30 Countries
• Adverse Events Reports: +10M
• Authorities Safety Alerts: +7k from more than 10 countries
• Approvals & Registrations: +5M
and much more!
.
|
Additional essential resources:
• Daily, Weekly, Yearly Reports
• Insights for PMS
• Rankings
• Data Predictions
• Trends
|
Why MedBoard ? Changing People Lives
The Growing and Long-Term Problem that MedBoard.com Solves
 |
 |
|
1. An extraordinary increased flow of news, information and data shared from key sources (e.g. authorities, clinical, manufacturers) that require planning, collecting and reviewing. In addition, there is a growing challenge of ensuring trusted sources and information. Increasing requirements and trends Fast face changes |
2. A significant increased demand for data, information, and evidence from key stakeholders (e.g. authorities, payers) and business processes, and new larger and more complex requirements for documentation, reports, PMS, literature, and data analysis.
|
This equation is growing in both sides, causing:
Increasing Manual, Repeated and Tedious work, and pressure on Professionals
Increasing Time spent in keeping up to date
Increasing searching time
Increasing time reviewing and reporting
Increasing Costs
Increasing Resources, time and budget
Limited Resources, time and budget
Increasing Risk for Organizations
Lack of time and resources to track and learn
Where current solutions are still:
Manual processes
Old Webs with PDFs and poor searching features
Social Media
Dispersed Official and Expert information
Very expensive resources or packages
Does this Sound Familiar to You? if YES, contact us, we can help you!
We have been solving this problem to our customers since 2018
 Value Propositions
Value Propositions
|
Powerful InnovationBuilt with state of the art technology and the most advanced data science, and using robust, secure and reliable infrastructures as .NET and AWS. Super-fast cloud computing capabilities running the core of the platform, assisted by Data Science and our unique classifiers, medical internal knowledge, and R&D innovation.
|
ConvenienceWe aim to be the world’s largest medical technology platform so you can find anything you need, instantly, at MedBoard, so you can maximise your success and positive impact to patients. Furthermore, convenience goes beyond that for us, it also means providing this with user-friendly interface, simplicity and great usability.
|
|
Customer FocusWe are a very customer-centric organization, driven constantly by our customers’ needs and our passion for invention. We put customers in the center, what you see today in MedBoard, it is the output of all requests made by our customers. Meeting customers’ needs is what we have been doing since day 1.
|
Price & SavingsOur focus is to offer a great value and product with accessible pricing. By using MedBoard, you are already saving costs and resources compared to doing nothing or any other alternative. You can get started on MedBoard with a low and predictable price, and scale up as your needs require. |
A Powerful Big Data Platform delivered in easy interfaces
What you see:
Simple and Easy
 |
A fundamental aspect of our vision at MedBoard is to create a platform that provides an intuitive user interface with seamless navigation and access to information. In order to deliver on this vision, our MedBoard team works closely with customers to continuously provide new exciting interfaces, features, applications and tools, to create a platform that is both visually dynamic and customizable.
|
Behind the curtains:
Big Machine
 |
MedBoard collects and organizes thousands of information and data per day, some of them very complex. At its core, it is a big data platform. Data Science, coding, algorithms, mathematical functions, all work like clockwork precision to deliver information at your fingertips, but also giving you the power and freedom to customize it and perform your tasks without any restrictions, it is just you and the data and information. |
Validated and Evaluated by top experts in the industry
Our software has been evaluated by a great number of experts in the industry, which continue to collaborate or work with us. In addition, more formal external evaluations have been taking place and for example, we are proud to have obtained a Digilab Certificate "Certificate for Regulatory Software" issued by the German Company Metecon .
In addition, we offer to medical manufacturers Validation packages and ready-to-use documentation (IQ,OQ,PQ) for the Built-in Modules:
If you are a medical device manufacturer and you are intending to use the Modules (e.g. Surveillances for Literature or PMS purposes) within your QMS and you require validations we are here to support too. Validating the software is very important to do, but it requires additional work and can be tedious, time consuming and expensive. This is why we do this work for you and we provide you a validation package with ready-to-use documentation and templates (IQ,OQ,PQ) and provide you ongoing support.
Awards & Achievements
In our short life we have already won awards and achieved milestones that we are super proud of!
MedBoard is proud to have collaborated with MHRA and UK Government and have helped within the process of the Covid-19 ventilator challenge in 2020. MedBoard task was to rapidly analyse millions of historical PMS data related to Mechanical ventilators (FSCAs, Adverse events, etc) in our platform, and draw patterns using different techniques, delivering detailed summaries and statistics, providing valuable insights into potential failure modes for new ventilators, in order to build next steps and actions.
This shows how trusted is MedBoard in Industry. Learn more about our contribution in this article here.
|
What a night! MedBoard won the prestigious TOPRA Innovation Award ahead of Merck & Co, Inc (USA). We are extremely proud of this award, as it was a joint nomination by many of our customers. This award is for them!
|
Our Journey
 May 2018 - First MedBoard data prototype platform was launched in collaboration with medical companies and experts. We validate our idea and engage with organizations and understand what are the main needs and market. We obtain very valuable insights and inputs that will be key in the future success. Ivan, Javi and the rest of the original team starts working on the big data platform while continuously developing the prototype to support customers. Significant investment is put into the validated idea while continuously gathering more inputs and requests from our current and new customers.
May 2018 - First MedBoard data prototype platform was launched in collaboration with medical companies and experts. We validate our idea and engage with organizations and understand what are the main needs and market. We obtain very valuable insights and inputs that will be key in the future success. Ivan, Javi and the rest of the original team starts working on the big data platform while continuously developing the prototype to support customers. Significant investment is put into the validated idea while continuously gathering more inputs and requests from our current and new customers.
 May 2020 - Current Big Data MedBoard platform is released. We get great feedback from companies and customers, we receive continuously requests from customers to add more features and content, time to deliver!
May 2020 - Current Big Data MedBoard platform is released. We get great feedback from companies and customers, we receive continuously requests from customers to add more features and content, time to deliver!
 May 2022 - MedBoard Platform achieves 50 Million resources in the search engine. Company achieves key number in customers and sales growth. We keep growing organically and are already beyond breakeven, most of our new customers (80%) come from referral. We continue heavily investing in R&D and development.
May 2022 - MedBoard Platform achieves 50 Million resources in the search engine. Company achieves key number in customers and sales growth. We keep growing organically and are already beyond breakeven, most of our new customers (80%) come from referral. We continue heavily investing in R&D and development.
Still MUCH MORE to do to meet our VISION!
Integrations and Partnerships
We collaborate with top class organizations in the medical industry to raise awareness of research, intelligence, automation and the importance of accessing up to date quality data, but also helping with newsletters, reports, and many more.
 |
 |
 |
Contact us to become a partner and let's work together on the VISION to make trusted information accessible |
Interested in Licensing MedBoard Data?
|
With Data Licensing and APIs, whether you have your own system or platform, RIM, or QMS, you can use our data to bring the best in class data to your customers. |








 Advanced Profiles & Countries Information
Advanced Profiles & Countries Information Dedicated Databases with Advanced Search features and Systematic Review
Dedicated Databases with Advanced Search features and Systematic Review News, Analytics, Insights, Reports
News, Analytics, Insights, Reports



