Databases & Resources ready for Regulatory Intelligence and Compliance monitoring
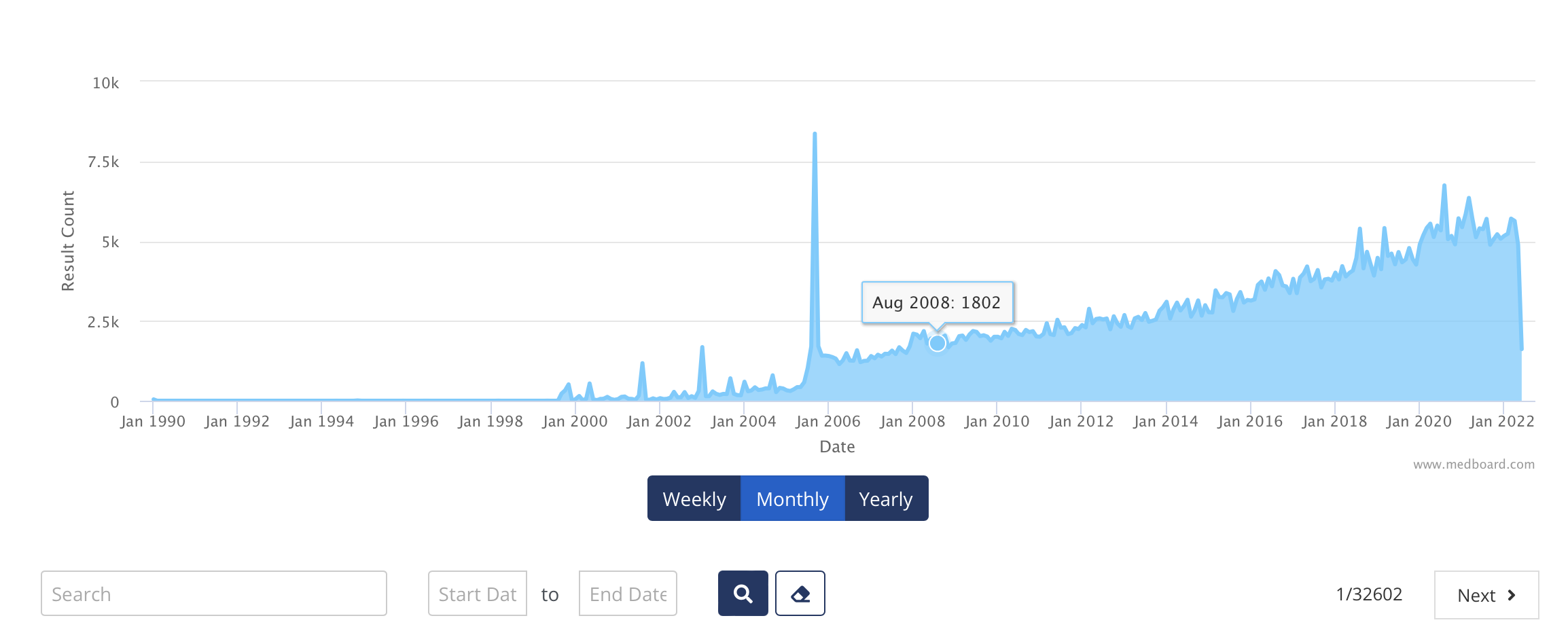
Databases with curated data, organised, classified, filters, trend visuals, attachments, translations, and many more features, ready to use and continuously UP TO DATE.
For Medical Devices, In Vitro Diagnostics and Medicines:
• Technical Standards Databases with lifecycle updates and adoptions: +50k Updates
• Regulatory Tools and Resources: + 500k Updates
• Inspections & Warning Letters: +50k Updates
Customize, Automate and Perform Continuous Systematic Review and Reporting
Our 'Surveillances' Built-In Module and Integrated with Databases allows users and organizations to not only automate results but also perform systematic review and reporting very easy.
|
Fully Customizable, Keywords and Sources. Combine Searches for Projects. |
Systematic Review & Appraisal, Reasons for exclusions, and scoring system.
|
Automated new results matching your query. Continuous review. |
Automated Flowchart Reporting |
Collaborative. Audit Trail and CFR Part 11 compliant |