This is an additional Add-on service for medical manufacturers and product developers, a secure, private repository and cloud database to keep track of their Portfolio and product Registrations in all countries around the globe. This module keeps track of key information to have all information up to date in a centralized and secure location where any member of your company can access to any time. Easy to use, fast and robust.
Once the information is uploaded, you can easily manage the portfolio:
- Search in any field in a fast way
- Filter by any Column, or field, and by Product Code, Basic UDI-DI, and Product Family
- Modify the information associated with it
- Export information (one code, all individual selected codes or all codes)
- Remove it (one code or remove all)
- Add Country Registrations related to the product.
- Account Manager provides security access levels.
- Etc.
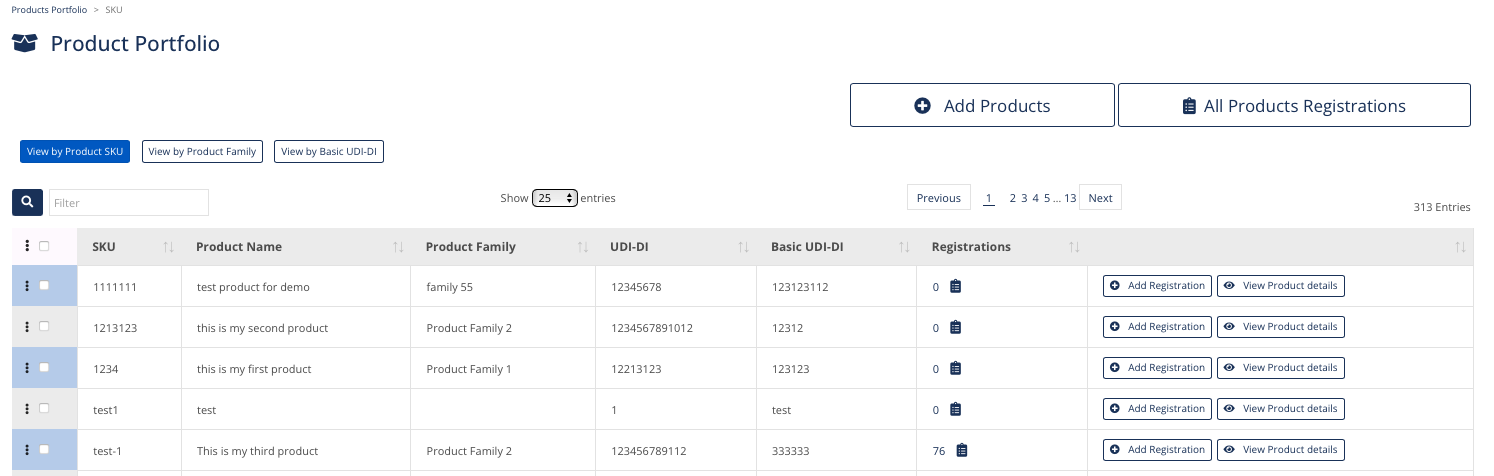
Products Portfolio
Build and Manage your Portfolio automatically
by Family, Basic UDI and Product Code
Add information related to your product
Link Product Codes to Registrations automatically
Provide visibility to the company
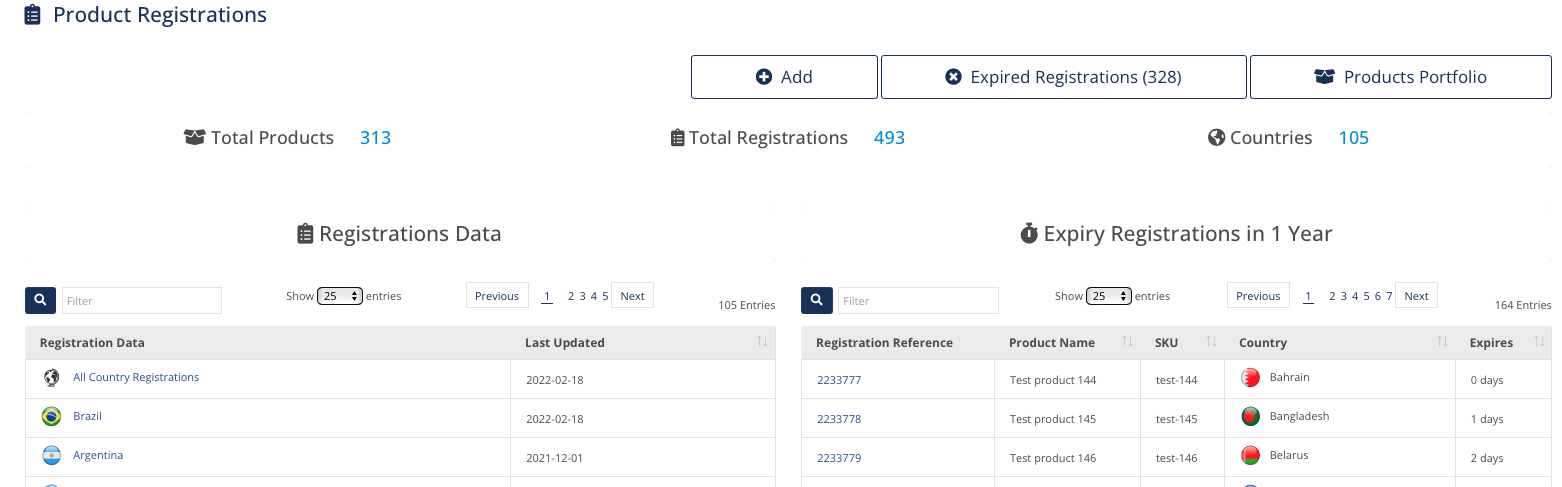
Products Registrations
.
Build and Manage your Registrations automatically
by Family, Basic UDI and Product Code
Add information related to your Registrations
.
Track registrations globally and by country
Use Regulatory and Operations flags
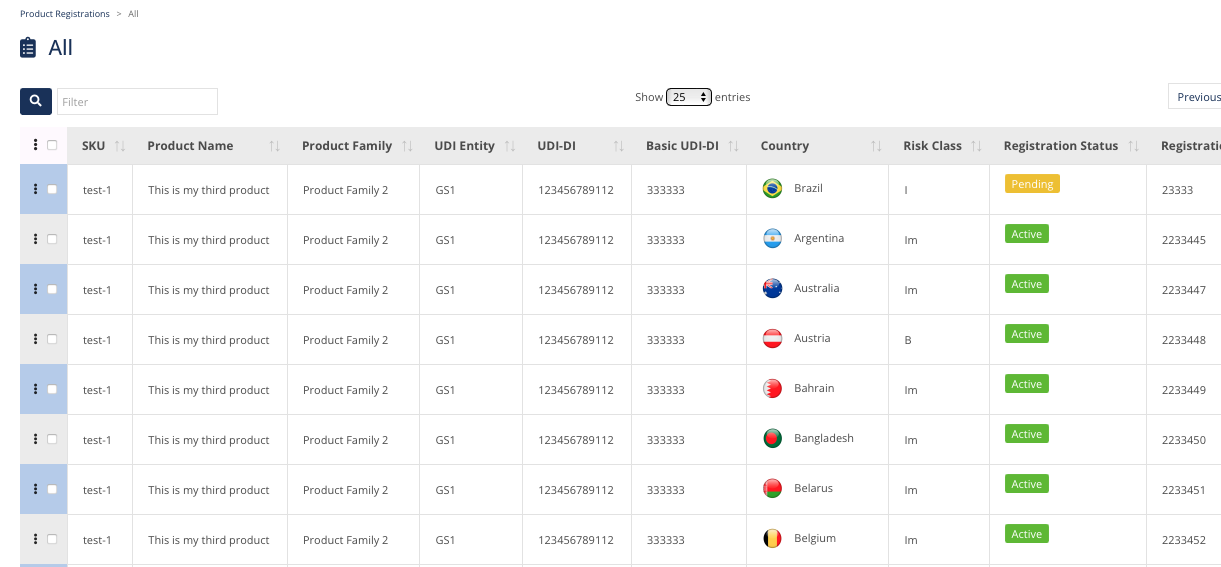
Some Data Fields include:
SKU-Product Code
Product Name
Product Family
UDI entity
UDI-DI
Basic UDI-DI
Country
Risk Classification Class
Registration Status
Registration / Certificate Reference
Country Authorized Representative
Country Distributor
Expiry Date of Registration
Regulatory Flags / Selling Status
Are you still doing this manually? if YES, contact us, we can help you!

