Databases ready for PMS & Vigilance
Databases with curated data, organised, classified, filters, trend visuals, attachments, translations, and many more features, ready to use and continuously UP TO DATE.
For Medical Devices, In Vitro Diagnostics and Medicines:
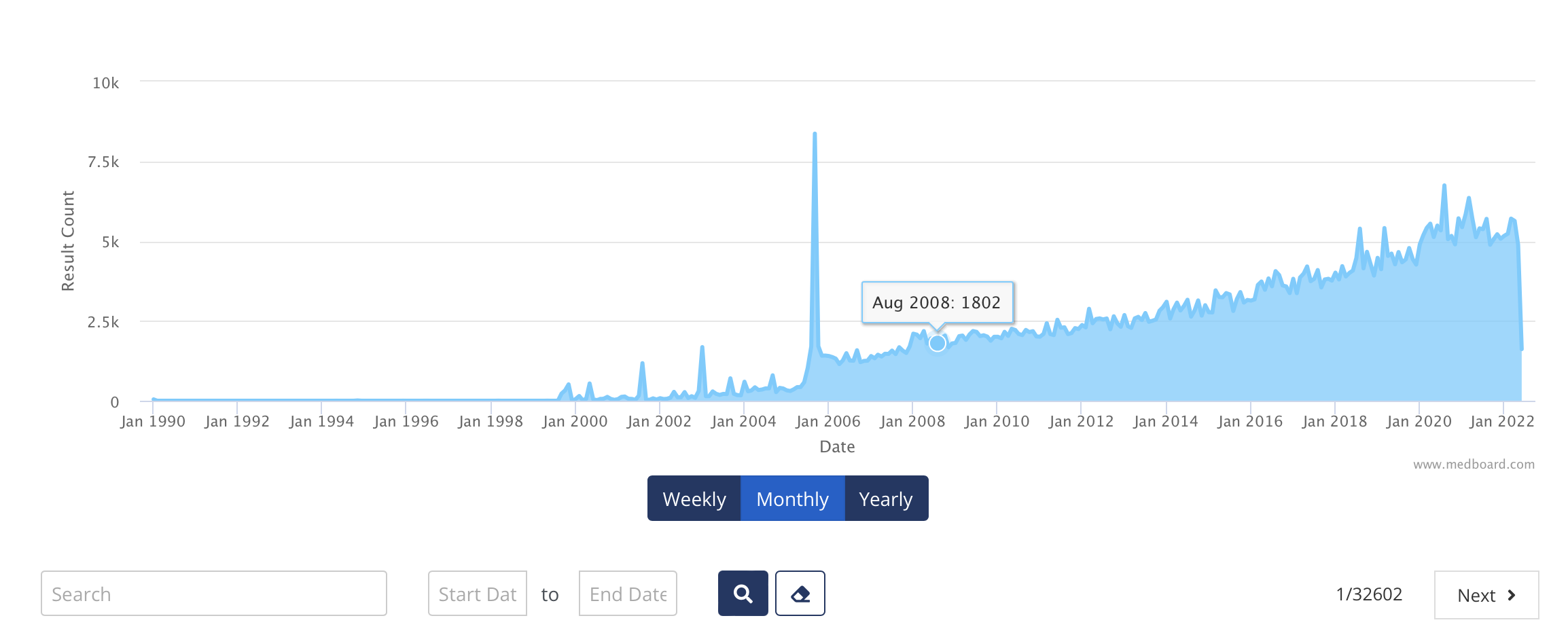
About PMS & Vigilance in the medical industry
This is the first and only end-to-end full customized PMS & Vigilance Solution on the market, helping organizations to save huge amount of resources and budget.
With the new increased requirements on Post-Market Surveillance, specially from EU MDR 2017/745 and EU IVDR 2017/746, and latest updates from key technical standards as ISO 14971:2019, they are very prescriptive about few points:
- The need to have a PMS Plan, with a very well defined plan and collection process.
- The need to analyse external sources for subject device information.
- The need to analyse external sources for equivalent / comparable devices information.
- The need to have a system which is: systematic, active, continuous and consistent.
The alternative to MedBoard is doing searches into hundreds of websites, for each product line with the applicable keywords, and make sure by evidence confirmation that whoever executes these searches always do it systematically and consistently (always with same exact keywords, same sources, same inputs, same process). The time and resources, and therefore costs, to execute this can be very high to implement and to execute it periodically every month, quarter, and year.
Automate and Perform Continuous Systematic Review and Reporting
Our Built-In Module and Integrated with Databases allows users and organizations to not only automate results but also perform systematic review and reporting.
|
Fully Customizable, Keywords and Sources. Combine Searches for Projects. |
Systematic Review & Appraisal, Reasons for exclusions, and scoring system.
|
Automated new results matching your query. Continuous review. |
Automated Flowchart Reporting. Screening, Eligibility and Appraisal steps. |
Collaborative. Audit Trail and CFR Part 11 compliant |